Abstract
Cathepsin G (CG) is a serine protease that is normally contained within primary granules of neutrophils and is expressed during the promyelocyte stage of myeloid cell development. We reported CG to be highly expressed in acute myeloid leukemia (AML) blasts compared with normal myeloid progenitors and leukocytes. We also showed CG to be a promising immunotherapeutic target in AML, having eluted a CG-derived peptide, FLLPTGAEA (hereafter referred to as CG1), from the surface of primary HLA-A*0201 (HLA-A2+) AML blasts and AML cell lines. Additionally, we showed CG expression in acute lymphoblastic leukemia (ALL) through endogenous expression by some ALL subsets and by cross-presentation from the marrow microenvironment. We also showed that CG1-specific cytotoxic T-lymphocytes (CTL) killed HLA-A2+ primary AML blasts and cell lines, but not normal myeloid progenitor cells. Furthermore, we detected CG1-CTL in peripheral blood mononuclear cells (PBMC) from AML patients following allogenic stem cell transplant. Here we report on the engineering, preclinical efficacy, and safety of CG1-targeting chimeric antigen receptor (CAR) T cells, which employ a novel T cell receptor-like (TCR-L) CAR construct that binds cell surface CG1/HLA-A2.
Methods Time-of-flight mass spectrometry was used to quantify CG1 expression on the cell surface. The antibody targeting CG1/HLA-A2 was developed by immunizing Harbour Mice with CG1/HLA-A2 complex, FACS-sorting of single B cells, screening B cell antibody-containing supernatant for binding to CG1/HLA-A2, and fusion cloning of VH and VL sequences from positively screened B cells. OCTET BLI assays were used to determine the binding of different alanine substituted CG1 monomers to lead antibodies. The affinity of each monomer was calculated based on BLI data. T2 binding assays were used to validate the binding of antibody monomers to cell surface CG1/HLA-A2 complexes. Immunohistochemistry (IHC) using human fresh and frozen PBMC and marrow was used to show the specificity of anti-CG1/HLA-A2 antibodies.
CG1 ScFV was cloned into pORBIT-LENTI-IRES-EGFR-III vector co-expressing either CD28 (CG1-CD28-CAR) or 4-1BB (CG1-41BB-CAR). HLA-A2+ normal donor (ND) T- cells were activated with anti-CD3 and anti-CD28 antibodies before lentiviral transduction with CG1 CAR constructs. Transduced cells were expanded and then characterized in vitro to determine CAR T quality. In vitro flow cytometry cytotoxicity assays were used to confirm in vitro killing of leukemia by CG1-CAR T cells. NSG mice engrafted with AML cell lines and AML patient-derived xenografts (PDX) were used to demonstrate the in vivo efficacy/pharmacokinetics/pharmacodynamics of CG1-CAR T cells from different donors.
Results We eluted CG1 peptide from the cell surface in 7 of 8 HLA-A2+ myeloid leukemia cell lines. CG1 was absent on the cell surface of HLA-A2+ non-myeloid solid tumor and lymphoid cell lines, as well as in normal HLA-A2+ neutrophils and PBMC.
Screening of different antibody clones for binding to CG1 peptide with different alanine amino acid substitutions identified top 5 antibody candidates with high affinity (single to sub-digit nM) for CG1-HLA-A2. T2 binding assays demonstrated high binding specificity of these five CG1 antibodies to CG1 monomers. The lead antibodies for CAR-T development were selected based on high antibody binding affinities (0.14-5 nM), specificity for CG1/HLA-A2, and no binding to ND marrow and PBMC.
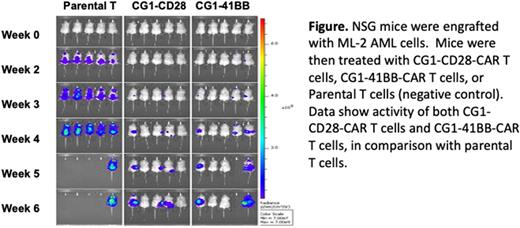
In vitro cytotoxicity assays confirmed in vitro killing of HLA-A2-transduced U937 (U937-A2) cells by CG1-CAR T cells. Both CG1-CD28- and the CG1-41BB-CAR constructs demonstrated high levels of killing. Lastly, in vivo AML mouse models using ML-2 and U937-A2 cell lines, and AML PDX validated the in vivo activity of CG1-CD28- and CG1-41BB-CAR T cells (Figure).
Conclusion Targeting CG1 using immunotherapy appears to be a valid approach in the setting of AML. We first showed that CG was highly expressed and presented on AML blasts. We developed TCRL-scFv that targets CG1/HLA-A2 on the cell surface and generated two different CAR constructs based on the scFv antibody sequence. We then showed potent anti-leukemia activity and specificity of CG1-CAR T cells against human leukemia in vitro and in vivo. Together, these data provide the pre-clinical proof of concept work that lays the foundation for further development of CG1-CAR T cells as a potential immunotherapy for patients with AML.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal